Understand how to prepare and administer once-monthly VIVITROL®
Watch the video for VIVITROL Injection Instructions
To ensure proper dosing, it is important to follow the preparation and administration instructions.
Preparation1
VIVITROL must be prepared and administered by a healthcare provider.
Remove the carton from refrigeration. Prior to preparation, allow drug to reach room temperature (approximately 45 minutes).
Inspect product
- Parenteral products should be visually inspected for particulate matter and discoloration prior to administration whenever solution and container permit. A properly mixed suspension will be milky white, will not contain clumps, and will move freely down the wall of the vial.
- VIVITROL must be suspended only in the diluent supplied in the carton and must be administered only with one of the administration needles supplied in the carton.
- The microspheres, diluent, preparation needle, and an administration needle with needle protection device are required for preparation and administration.
Choose the appropriate needle
Two thin-walled 1 1/2-inch needles with needle protection device and two 2-inch thin-walled needles with needle protection device have been provided to accommodate varying patient body habitus.
- For patients with a larger amount of subcutaneous tissue overlying the gluteal muscle, the administering healthcare provider may utilize the supplied 2-inch needle with needle protection device to help ensure that the injectate reaches the intramuscular mass.
- For very lean patients, the 1 1/2-inch needle may be appropriate to prevent the needle from contacting the periosteum. Either needle may be used for patients with average body habitus.
- A spare administration needle of each size is provided in case of clogging. Do not substitute any other components for the components of the carton.
Prepare the VIVITROL suspension using aseptic technique.
Keep out of reach of children.
Highlights of Administration1
VIVITROL must be prepared and administered by a healthcare provider.
Administer the VIVITROL suspension using aseptic technique.
Prior to initiation of VIVITROL, an opioid-free duration of a minimum of 7-10 days is recommended for patients, to avoid precipitation of opioid withdrawal that may be severe enough to require hospitalization.
The recommended dose of VIVITROL is 380 mg delivered intramuscularly deep as a gluteal injection every 4 weeks or once a month, alternating buttocks for each subsequent injection, using the carton components provided.
Pre-treatment with oral naltrexone is not required before using VIVITROL.
VIVITROL must ONLY be administered as a deep intramuscular gluteal injection.
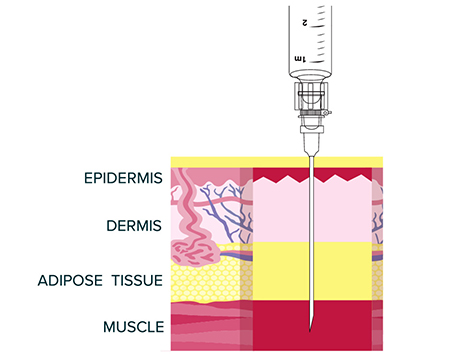
Remember to aspirate for blood before injection. If blood aspirates or the needle
clogs, do not inject.
WARNING: To reduce the risk of a needlestick:
- Do not intentionally disengage the needle protection device
- Discard bent or damaged needle into a sharps container and use the spare needle provided. Do not attempt to straighten the needle or engage needle protection device if the needle is bent or damaged
- Do not mishandle the needle protection device in a way that could lead to protrusion of the needle
- Do not use free hand to press sheath over needle
Injection site reactions1
- VIVITROL injections may be followed by pain, tenderness, induration, swelling, erythema, bruising, or pruritus; however, in some cases injection site reactions may be very severe
- Injection site reactions not improving may require prompt medical attention, including, in some cases, surgical intervention
- VIVITROL is administered as a deep intramuscular gluteal injection, and inadvertent subcutaneous injection of VIVITROL may increase the likelihood of severe injection site reactions
- Select proper needle size for patient body habitus, and use only the needles provided in the carton
- Patients should be informed that any concerning injection site reactions should be brought to the attention of their healthcare provider
Interested in
Request a representative for
See Section 2.6 of full Prescribing Information for important Dosage and Administration information and complete Directions for Use.